DALTON’S LAW AND THE GIBBS-DALTON LAW
Dalton’s law shows that for a mixture of gases occupying a given volume at a certain temperature,
the total pressure of the mixture is equal to the sum of the partial pressures of the constituents of the
mixture, i.e.,
The partial pressure exerted by each constituent in the mixture is independent of the existence of
other gases in the mixture. Figure 2.1 shows the variation of mass and pressure of dry air and water
vapor, at an atmospheric pressure of 14.697 psia (101,325 Pa) and a temperature of 75°F (23.9°C).
The principle of conservation of mass for nonnuclea
r processes gives the following relationship:
Applying Dalton’s law for moist air, we have
Dalton’s law is based on experimental results. It is more accurate for gases at low pressures.
Dalton’s law can be further extended to state the relationship of the internal energy, enthalpy, and
entropy of the gases in a mixture as the Gibbs-Dalton law:




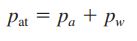



Comments
Post a Comment